Chemistry class 9
1.1 Padarth aur unki sanrachna (पदार्थ और उनकी संरचना)
Is adhyay mein hum padarth aur unki sanrachna ke baare mein jaanenge. Padarth jiske hum log prakriti ko bhi kehte hain, unka vastavik swaroop, unke gun aur unke anukool vishestayein iss adhyay mein batayi gayi hain.
1.2 Padarth ki paribhasha (पदार्थ की परिभाषा)
Padarth ek vastu hoti hai jo hamare charo aur payi jati hai. Ye vastu siddh hone par ham use padarth kehte hain. Padarth ki visheshta ye hai ki ye vastu gunon se paripurn hoti hai.
1.3 Padarthon ke prakar (पदार्थों के प्रकार)
Iss adhyay mein ham padarth ki pramukh 3 prakar – dravya, tatva aur mishran ke baare mein padhenge. Dravya ek aisa padarth hai jo apne aap mein sthir hota hai, jabki tatva sabse chote bhag hote hain aur mishran keval do ya adhik padarthon ke mishran hote hain.
1.4 Dravya (द्रव्य)
Dravya ke prakar aur unki visheshtayein iss adhyay mein batayi gayi hain. Dravya ko dhatu, adhatu aur mishrit dhatu ke roop mein bhi vargikrit kiya ja sakta hai. Har ek dravya ki apni apni visheshtayein hoti hain.
1.5 Tatva (तत्व)
Tatva ek aisa padarth hai jo dravya ke sabse chote bhag hote hain. Inme kuchh pramukh tatva – hydrogen, helium, carbon, oxygen, nitrogen aadi hote hain. Tatva ke gunon ke anusaar unhein alag-alag kram mein rakha ja sakta hai.
1.6 Mishran (मिश्रण)
Mishran do ya adhik padarthon ke mishran se banta hai. Iss adhyay mein mishran ki visheshtayein aur unke prakar ke baare mein bataya gaya hai. Mishran ke prakar – upyogi mishran, anupyogi mishran aur ubhaya mishran hote hain.
Introduction
NCERT ka yeh adhyay humein batata hai ki hamare aas-paas jo bhi vastuyen hain, jaise ki alag-alag akar, aakaar aur sparsh ke saath – sabhi padarthon ko ek samaan naam diya gaya hai jise “padarth” kehte hain. Hamari sansaar ki har vastu padarth se bani hai – hawa jo ham saans lete hain, khana jo ham khate hain, patthar, baadal, tare, paudhe aur jaanwar, ya phir ek chhota sa boond paani ya ek ret ke kanse – har cheez padarth hai. Aur jab ham aas-paas dekhte hain toh ham dekhte hain ki saari cheezen jinke baare mein hum baat kar rahe hain, wo sab ek sthan par baste hain aur unke paas maasa aur avlum dono hota hai. Hamari duniya ke prati kiya gya adhyayan hamein yeh batata hai ki sabhi vastuon ke andar maasa* aur avlum** dono hota hai.
Matter
In chemistry, matter refers to anything that has mass and takes up space. This includes everything from the air we breathe, the water we drink, and the substances that make up our bodies, to the objects around us, such as tables, chairs, and buildings. Matter can exist in different forms, including solid, liquid, gas, and plasma, and can undergo physical and chemical changes.
Chemistry is the study of matter, its properties, and its behavior. Chemists use their knowledge of the properties of matter to study and manipulate substances, to create new materials and compounds, and to understand the chemical reactions that occur in living and non-living systems.
Let’s start a chapter
- Human beings have always been curious about their surroundings and have attempted to understand them.
- Early Indian philosophers classified matter into five basic elements known as the “Panch Tatva” – air, earth, fire, sky, and water.
- According to them, everything, whether living or non-living, was composed of these five basic elements.
- Ancient Greek philosophers also had a similar classification of matter.
- Modern scientists have developed two types of classification of matter based on physical properties and chemical nature.
- In this chapter, we will learn about matter based on its physical properties, which include characteristics such as mass, volume, density, and shape.
- Physical properties of matter can be observed or measured without changing the identity of the substance.
- Chemical aspects of matter, which deal with the composition, properties, and reactions of substances at the molecular level, will be discussed in later chapters.
- Understanding the properties of matter is crucial in various fields such as engineering, medicine, and environmental science.
1.1 Physical Nature of Matter 1.1.1 MATTER IS MADE UP OF PARTICLES
- Two schools of thought existed for a long time regarding the nature of matter.
- One school believed matter to be continuous, similar to a block of wood or a piece of clay.
- The other school believed that matter was made up of particles like sand or grains of salt.
- To determine the nature of matter, an activity can be performed.
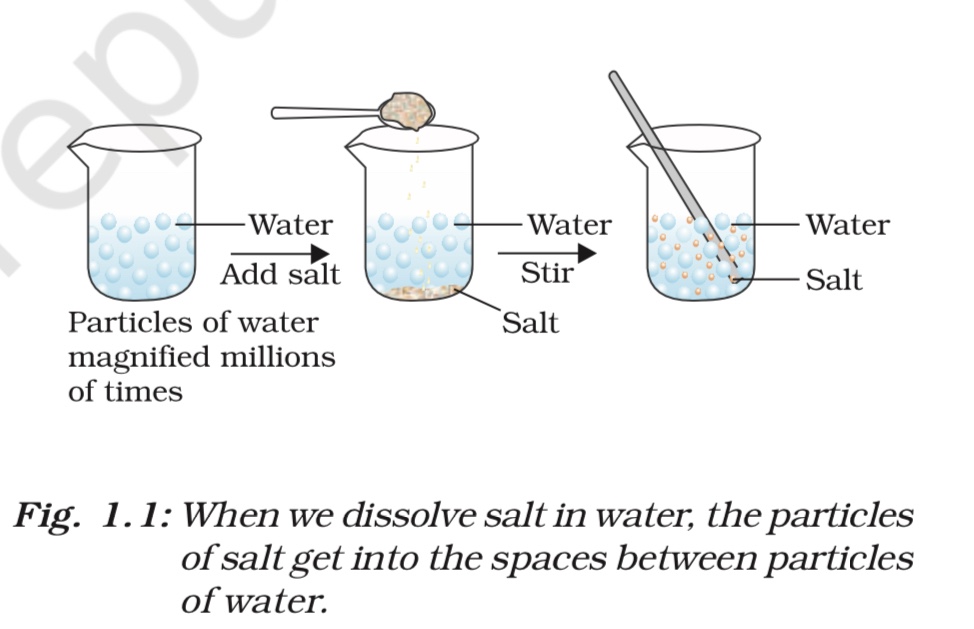
- In the activity, a small amount of sugar or salt can be added to a glass of water and stirred until it dissolves.
- If matter is continuous, the sugar or salt should mix uniformly with the water, forming a homogeneous mixture with no visible particles.
- However, if matter is particulate, the sugar or salt particles should be visible as they float in the water, forming a heterogeneous mixture.
- The observation of visible particles would support the idea that matter is made up of particles rather than being continuous.
- This activity helps in understanding the nature of matter and supports the concept of the particulate nature of matter.
Activity ______________1.1 • Take a 100 mL beaker. • Fill half the beaker with water and mark the level of water. • Dissolve some salt/ sugar with the help of a glass rod. • Observe any change in water level. • What do you think has happened to the salt? • Where does it disappear? • Does the level of water change? Please solve the activity and explain
- In this activity, we take a 100 mL beaker and fill half of it with water, then mark the level of water.
- Next, we dissolve some salt or sugar in the water using a glass rod.
- As we observe the mixture, we may notice that the salt or sugar particles are no longer visible, and they appear to have disappeared.
- This disappearance of the salt or sugar particles can be explained by the particulate nature of matter.
- The individual salt or sugar particles have mixed with the water particles and have become too small to be seen individually.
- The level of water in the beaker does not change, indicating that the dissolved salt or sugar did not add or remove any water from the beaker.
- This activity demonstrates the concept of the particulate nature of matter and how substances can dissolve in water without changing the overall volume of the mixture.
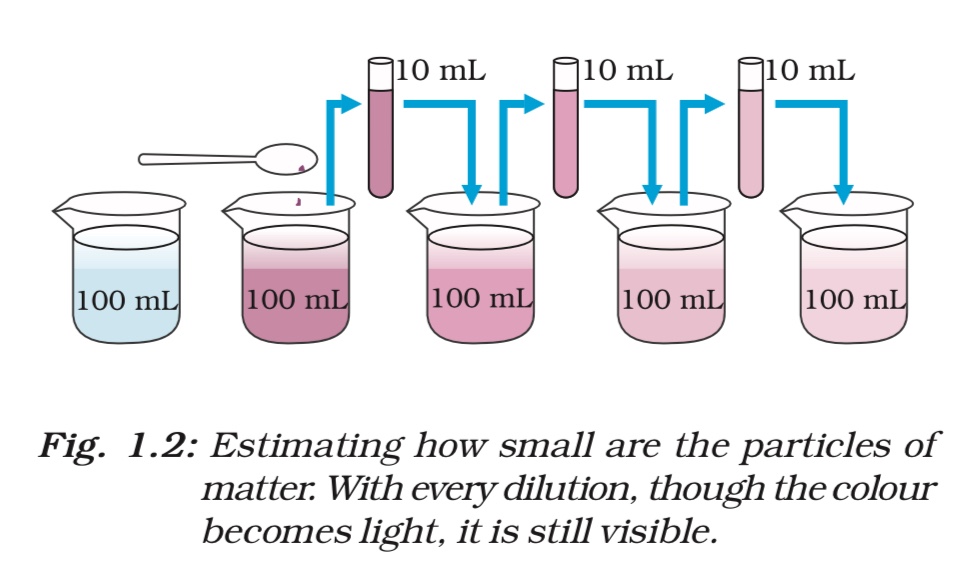
Activity ______________1.2 • Take 2–3 crystals of potassium permanganate and dissolve them in 100 mL of water. Take out approximately 10 mL of this solution and put it into 90 mL of clear water. • Take out 10 mL of this solution and put it into another 90 mL of clear water. • Keep diluting the solution like this 5 to 8 times. • Is the water still coloured ?
Here are the steps involved in this activity:
- Take 2-3 crystals of potassium permanganate and dissolve them in 100 mL of water to obtain a concentrated solution.
- Take out approximately 10 mL of this solution and put it into 90 mL of clear water, diluting the solution.
- Repeat the process of taking out 10 mL of the diluted solution and putting it into another 90 mL of clear water, diluting it further.
- Repeat the above step 5 to 8 times, each time diluting the solution further.
- Observe the color of the solution after each dilution.
- As we dilute the solution, the intensity of the purple color of the potassium permanganate solution gradually becomes lighter and lighter until it is no longer visible to the naked eye.
This activity demonstrates the concept of dilution and how it can be used to make a solution less concentrated. It also shows how the intensity of color in a solution decreases as the concentration of the colored substance decreases through dilution.
This activity, which involves diluting a solution of potassium permanganate or Dettol, demonstrates the concept of the particle nature of matter.
Here are the key points to note and explain:
- The activity shows that a few crystals of potassium permanganate or a small amount of Dettol can color or scent a large volume of water (about 1000 L), indicating that there must be millions of tiny particles in just one crystal or drop of the solution.
- As the solution is diluted, the concentration of the particles in the solution decreases, leading to a decrease in the intensity of color or scent.
- This implies that matter is not continuous, but is made up of tiny particles, which keep dividing themselves into smaller and smaller particles, as seen in the case of potassium permanganate crystals.
- This particle nature of matter is a fundamental concept in the study of chemistry and physics.
- This activity can be used to explain other phenomena related to particle nature of matter, such as diffusion, Brownian motion, and osmosis.
- In addition to potassium permanganate and Dettol, other substances can also be used to demonstrate the particle nature of matter, such as ink, food coloring, or even sugar or salt.
The particles of matter are very small – they are small beyond our imagination!!!!
1.2 CharacteristicsofParticlesof Matter
1.2.1 PARTICLES OF MATTER HAVE SPACE BETWEEN THEM
Notes
Matter is composed of particles that have space between them. When we dissolve substances like sugar, salt, or Dettol in water, the particles of these substances get evenly distributed throughout the water. This is because there is enough space between the particles of water for the particles of the dissolved substance to fit in. Similarly, when we make tea, coffee, or lemonade, particles of the flavoring substances get into the spaces between the particles of the water molecules. This is evidence that particles of matter have space between them.
Explanation:
All matter is made up of tiny particles, such as atoms or molecules, that are constantly in motion. These particles have space between them, which allows them to move around freely. When we dissolve a substance like sugar or salt in water, the particles of the substance mix with the particles of water. The particles of the dissolved substance are able to fit into the spaces between the water particles because there is enough space between them.
Similarly, when we make a flavored drink like tea, coffee, or lemonade, the particles of the flavoring substance mix with the particles of water. The flavoring particles are able to fit into the spaces between the water particles because there is enough space between them.
This concept of particles having space between them is important to understand because it helps us explain why certain phenomena occur. For example, the movement of air molecules is responsible for sound waves, and the space between particles in a solid material is responsible for its ability to expand and contract with changes in temperature.
1.2.2 PARTICLES OF MATTER ARE CONTINUOUSLY MOVING
Activity ______________1.3
- Put an unlit incense stick in a corner of your class. How close do you have to go near it so as to get its smell?
- Now light the incense stick. What happens? Do you get the smell sitting at a distance?
- Record your observations.
Notes
• When an unlit incense stick is placed in a corner of a classroom, one has to go close to it to get its smell. •
When the incense stick is lit, the smell of the incense can be detected even from a distance. •
This indicates that the particles of matter are continuously moving and spread out in all directions. •
The smoke released by the burning incense stick contains particles that are moving and spreading out in all directions, carrying the smell of the incense along with them. •
This phenomenon is due to the kinetic energy of the particles of matter.
Explanation:
When an unlit incense stick is placed in a corner of a classroom, one has to go close to it to get its smell. This is because the particles of matter in the air near the incense stick are not moving fast enough to carry the smell to a distance. However, when the incense stick is lit, the smoke released by the burning incense stick contains particles that are moving and spreading out in all directions, carrying the smell of the incense along with them. This allows the smell to be detected even from a distance.
This phenomenon is due to the kinetic energy of the particles of matter. All particles of matter, whether in a solid, liquid, or gas state, are in constant motion due to their kinetic energy. In the case of the incense stick, the heat from the flame causes the solid particles of the incense to turn into gas, which then spreads out in all directions. These gas particles collide with the air particles and transfer their kinetic energy to them, causing them to move faster and spread out further.
This activity helps to demonstrate the concept of particle motion and diffusion, which is important in understanding many phenomena in nature. For example, the diffusion of gases is responsible for the movement of oxygen and carbon dioxide in and out of our lungs during respiration.
Activity ______________1.4
- Take two glasses/beakers filled with water.
- Put a drop of blue or red ink slowly and carefully along the sides of the first beaker and honey in the same way in the second beaker.
- Leave them undisturbed in your house or in a corner of the class.
- Record your observations.
- What do you observe immediately after
adding the ink drop? - What do you observe immediately after
adding a drop of honey? - How many hours or days does it take
for the colour of ink to spread evenly throughout the water?
Notes
• When a drop of blue or red ink is added to a glass of water, it immediately spreads out and starts to mix with the water.
• The ink color slowly spreads throughout the water over a period of hours or days, until it is evenly distributed.
• When a drop of honey is added to a glass of water, it does not mix with the water and remains as a droplet on the surface of the water.
• This is because honey is denser than water and its particles are not small enough to mix with the water.
• This activity demonstrates the concept of density and diffusion, which are important properties of matter.
Explanation:
When a drop of blue or red ink is added to a glass of water, it immediately spreads out and starts to mix with the water. This is because the particles of ink are smaller than the particles of water, and they are able to move and mix with the water particles due to diffusion. Over a period of hours or days, the ink color slowly spreads throughout the water until it is evenly distributed.
On the other hand, when a drop of honey is added to a glass of water, it does not mix with the water and remains as a droplet on the surface of the water. This is because honey is denser than water, and its particles are not small enough to mix with the water particles. The honey droplet remains on the surface due to the surface tension of the water.
This activity helps to demonstrate the concept of density and diffusion, which are important properties of matter. Density is the measure of how much mass is packed into a given volume of a substance. Diffusion is the process by which particles of matter move from an area of high concentration to an area of low concentration until the concentration is equalized.
In summary, this activity helps to illustrate how the size and density of particles affect their ability to mix with other particles of matter, and how diffusion plays a role in the movement and distribution of particles in a liquid.
Activity ______________1.5
- Drop a crystal of copper sulphate or potassium permanganate into a glass of hot water and another containing cold water. Do not stir the solution. Allow the crystals to settle at the bottom.
- What do you observe just above the solid crystal in the glass?
- What happens as time passes?
- What does this suggest about the
particles of solid and liquid? - Does the rate of mixing change with
temperature? Why and how?
Notes
- • When a crystal of copper sulphate or potassium permanganate is dropped into a glass of hot or cold water and left undisturbed, the crystal settles at the bottom and starts to dissolve.
- • Just above the solid crystal in the glass, a gradient of color can be observed where the concentration of the dissolved substance is higher closer to the crystal.
- • As time passes, the gradient becomes less distinct, and the color becomes more evenly distributed throughout the water.
- • This suggests that the particles of the solid crystal are moving and mixing with the particles of the liquid water due to diffusion.
- • The rate of mixing is faster with hot water than with cold water due to the increased kinetic energy of the particles in hot water, which causes them to move and mix more rapidly.
Explanation:
When a crystal of copper sulphate or potassium permanganate is dropped into a glass of hot or cold water, it starts to dissolve, and the particles of the solid crystal start to mix with the particles of the liquid water due to diffusion. Just above the solid crystal in the glass, a gradient of color can be observed where the concentration of the dissolved substance is higher closer to the crystal. This gradient becomes less distinct as time passes, and the color becomes more evenly distributed throughout the water.
This activity demonstrates the concept of diffusion and the movement of particles in a liquid. The particles of the solid crystal dissolve and mix with the particles of the liquid water, and this mixing occurs due to the random motion of the particles, which causes them to move from areas of high concentration to areas of low concentration.
The rate of mixing is faster with hot water than with cold water due to the increased kinetic energy of the particles in hot water, which causes them to move and mix more rapidly. The higher temperature causes the particles of the liquid water to move faster and collide more frequently with the particles of the solid crystal, resulting in faster dissolution and mixing.
In summary, this activity helps to demonstrate how the temperature of a liquid affects the rate of mixing and diffusion of particles, and how the movement of particles can cause them to mix and distribute evenly throughout a liquid.
Notes
• Particles of matter possess kinetic energy, which increases with an increase in temperature.
• The intermixing of particles of two different types of matter on their own is called diffusion.
• Diffusion occurs due to the random motion of particles.
• Heating increases the kinetic energy of particles, which results in an increase in the speed of their motion and hence the rate of diffusion.
Explanation:
In the activities mentioned above, we observed that particles of matter intermix on their own with each other, getting into the spaces between particles. This intermixing of particles of two different types of matter on their own is called diffusion. Diffusion occurs due to the random motion of particles. As particles move randomly, they collide with other particles and transfer energy and momentum, resulting in intermixing.
The kinetic energy of particles increases with an increase in temperature. Heating provides more energy to particles, and they move faster due to increased kinetic energy. This increase in speed leads to more frequent and energetic collisions between the particles, which results in a faster rate of diffusion. Therefore, on heating, diffusion becomes faster.
In conclusion, the kinetic energy possessed by particles of matter leads to the phenomenon of diffusion. As the temperature increases, the kinetic energy of particles also increases, leading to an increase in the rate of diffusion.
1.2.3 PARTICLES OF MATTER ATTRACT EACH OTHER
Activity ______________1.6
- Play this game in the field— make four groups and form human chains as suggested:
- The first group should hold each other from the back and lock arms like Idu-Mishmi dancers
- The second group should hold hands to form a human chain.
- The third group should form a chain by touching each other with only their finger tips.
- Now, the fourth group of students should run around and try to break the three human chains one by one into as many small groups as possible.
- Which group was the easiest to break? Why?
- If we consider each student as a particle of matter, then in which group the particles held each other with the maximum force?
Notes
• The game involves forming three human chains using different methods.
• The fourth group tries to break the three human chains by running around.
• The ease of breaking the human chains is related to the strength of the interparticle forces holding them together.
Explanation:
In the game, the first group holds each other from the back and locks arms, forming a strong human chain. The second group holds hands to form a human chain, while the third group forms a chain by touching each other with only their fingertips, forming a weak human chain. The fourth group tries to break the human chains one by one into as many small groups as possible by running around them.
The easiest chain to break would be the third group, where the students are only holding each other with their fingertips, forming a weak human chain. The second group, where the students are holding hands, would be relatively stronger, and the first group, where the students are locking arms, would be the strongest human chain.
If we consider each student as a particle of matter, then the first group, where the students are locking arms, would be holding each other with the maximum force. This is because the locking of arms creates a strong interparticle force that holds the chain together. In contrast, the third group, where the students are holding each other with their fingertips, would have the weakest interparticle forces holding the chain together, and hence it would be the easiest to break.
In conclusion, the ease of breaking the human chains in the game is related to the strength of the interparticle forces holding them together. The stronger the interparticle forces, the harder it is to break the chain. If we consider each student as a particle of matter, then the group with the maximum force would be the one where the students are holding each other with the maximum force, i.e., the group where the students are locking arms.
Activity ______________1.7
- Take an iron nail, a piece of chalk and a rubber band.
- Try breaking them by hammering, cutting or stretching.
- In which of the above three substances do you think the particles are held together with greater force?
Notes
• The activity involves testing the strength of three substances, i.e., an iron nail, a piece of chalk, and a rubber band.
• The objective is to determine which substance has particles held together with greater force.
Explanation:
In the activity, we are given an iron nail, a piece of chalk, and a rubber band. We are supposed to test the strength of these substances by hammering, cutting, or stretching them.
When we try to break the iron nail by hammering or cutting, we observe that it is difficult to break it. This is because the particles in the iron nail are held together with a strong force of attraction, called metallic bonding. This bonding is very strong, and hence the iron nail is very tough and durable.
When we try to break the piece of chalk by hammering or cutting, we observe that it is relatively easy to break it. This is because the particles in chalk are held together with weaker forces of attraction, called van der Waals forces. These forces are weaker than metallic bonding and hence the piece of chalk is not as strong as the iron nail.
When we try to break the rubber band by stretching it, we observe that it is relatively easy to break it. This is because the particles in the rubber band are held together with weak forces of attraction, called intermolecular forces. These forces are weaker than van der Waals forces and hence the rubber band is not as strong as either the iron nail or the piece of chalk.
Therefore, we can conclude that the particles in the iron nail are held together with the greatest force, followed by the particles in the piece of chalk, and then the particles in the rubber band. The strength of the interparticle forces determines the strength of the substance, and the stronger the forces, the more difficult it is to break the substance.
Activity ______________1.8
- Take some water in a container, try cutting the surface of water with your fingers.
- Were you able to cut the surface of water?
- What could be the reason behind the surface of water remaining together?
Notes
• Take some water in a container.
• Try cutting the surface of water with your fingers.
• The surface of water does not get cut.
Explanation:
The surface of water has a property called surface tension which enables it to resist external forces. The particles on the surface of water experience a net inward force due to the unbalanced attractive forces between the water molecules. This force causes the surface of water to contract and become like an elastic sheet. This is why the surface of water does not break when we try to cut it with our fingers.
This surface tension property of water is responsible for many phenomena, such as the formation of droplets, capillary action, and the ability of some insects to walk on water.
Questions:
Q1. Which of the following are matter?
Chair, air, love, smell, hate, almonds, thought, cold, lemon water, smell of perfume.
- Give reasons for the following observation:
The smell of hot sizzling food reaches you several metres away, but to get the smell from cold food you have to go close. - A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
- What are the characteristics of the particles of matter?
MATTER IN OUR SURROUNDINGS
Answers:
A1. Matter: Chair, air, almonds, cold, lemon water, smell of perfume. Not matter: Love, smell, hate, thought.
A2. When the temperature of the food increases, the kinetic energy of the particles of the food also increases, causing them to move faster and spread out. This leads to the diffusion of the smell to a greater distance. In the case of cold food, the particles have lower kinetic energy, so they move slower and are not able to spread out as easily.
A3. This observation shows the property of matter called “viscosity”. Viscosity is the property of a fluid that resists the relative motion between its different layers. The water in the swimming pool has viscosity, which allows the diver to cut through it.
A4. Characteristics of particles of matter:
- Particles have space between them
- Particles are constantly moving
- Particles attract each other
- Particles have kinetic energy
- Particles are in constant random motion.
1.3 States of Matter
Properties of the three states of matter:
- Solids:
- Solids have a fixed shape and volume.
- Particles of solids are tightly packed and have strong intermolecular forces.
- Solids cannot be compressed easily.
- Solids are not very fluid and do not flow easily.
- Solids have a definite melting point and boiling point.
Examples of solids are iron, wood, stone, and ice.
- Liquids:
- Liquids have a fixed volume but no fixed shape.
- Particles of liquids are loosely packed and have weaker intermolecular forces than solids.
- Liquids can be compressed slightly.
- Liquids can flow and take the shape of the container in which they are kept.
- Liquids have a definite boiling point but no definite melting point.
Examples of liquids are water, milk, oil, and vinegar.
- Gases:
- Gases have neither fixed shape nor fixed volume.
- Particles of gases are far apart and have weak intermolecular forces.
- Gases can be compressed easily.
- Gases can flow and occupy the entire volume of the container in which they are kept.
- Gases do not have a definite melting point or boiling point.
Examples of gases are air, oxygen, carbon dioxide, and helium.
These are the general properties of the three states of matter. We can observe these properties in various substances around us.
1.3.1 THE SOLID STATE
Activity _ 1.9
- Collect the following articles — a pen, a book, a needle and a piece of wooden stick.
- Sketch the shape of the above articles in your notebook by moving a pencil around them.
- Do all these have a definite shape, distinct boundaries and a fixed volume?
- What happens if they are hammered,
pulled or dropped? - Are these capable of diffusing into each
other? - Try compressing them by applying
force. Are you able to compress them?
1.9 Notes
• The articles collected for the activity are a pen, a book, a needle, and a piece of wooden stick.
• Sketch the shape of the articles in a notebook by moving a pencil around them.
• All these articles have a definite shape, distinct boundaries, and a fixed volume.
• If they are hammered, pulled, or dropped, they retain their shape and do not deform easily.
• These are not capable of diffusing into each other.
• On compressing them by applying force, they do not compress easily, and their volume remains almost constant.
Explanation:
In this activity, we have observed the properties of solids. Solids have a definite shape, distinct boundaries, and a fixed volume. The particles of a solid are closely packed and held together by strong intermolecular forces. As a result, solids have a strong resistance to deformation and do not flow easily. Therefore, when we hammer, pull, or drop a solid, it retains its shape and does not deform easily.
Unlike liquids and gases, solids are not capable of diffusing into each other, as their particles are closely packed and do not have the freedom to move around. When we try to compress a solid by applying force, its volume remains almost constant as the particles are held closely packed and do not have much space to move.
Thus, the activity helps us understand the characteristics of solids and how they differ from the other states of matter.
Consider the following:
(a) What about a rubber band, can it change its shape on stretching? Is it a solid?
(b) What about sugar and salt? When kept in different jars these take the shape of the jar. Are they solid?
(c) What about a sponge? It is a solid yet we are able to compress it. Why?
(a) A rubber band can change its shape on stretching. It is a solid but has some properties of liquids. Such solids are called elastomers.
(b) Sugar and salt are also solids but they can flow slowly like liquids. Such solids are called amorphous solids.
(c) A sponge is a solid with small holes or pores in it. When we compress a sponge, its air-filled pores collapse, thus reducing its volume.